NICE appeal on anti-GD2 therapy
Solving Kids’ Cancer UK’s appeal to the National Institute for Health and Care Excellence (NICE) on the use of immunotherapy.
Background
The use of anti-GD2 immunotherapy is the most recent change to the standard frontline treatment pathway for high-risk neuroblastoma. Dinutuximab beta is the drug used in standard therapy in the UK. It is used as a maintenance (relapse prevention) therapy as the last stage of treatment and has shown notable improvements in the outcomes for children with high-risk neuroblastoma.
The introduction of dinutuximab beta into standard of care is in part thanks to the dedicated efforts of Solving Kids’ Cancer UK during the NICE consultation on the use of dinutuximab beta, and its predecessor, dinutuximab.
The National Institute for Health and Care Excellence (NICE) is a national body in the UK that evaluates new health technologies for NHS, taking into account clinical effectiveness as well as value for money. They provide the proper guidance on approved medicines that are used in NHS treatment.
NICE appraisal of dinutuximab
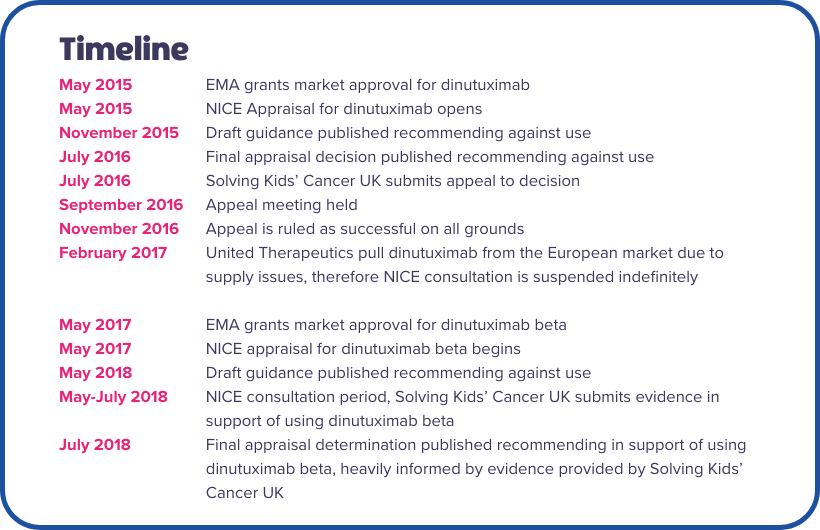
Dinutuximab is a type of anti-GD2 antibody therapy that is developed by United Therapeutics under the name Unituxin©. In May 2015, a NICE appraisal to assess the use of dinutuximab for high-risk neuroblastoma in standard NHS care was opened.
Following a draft recommendation against its use, and lengthy consultation period, NICE published its final appraisal decision (FAD) in July 2016, recommending that dinutuximab should not be introduced to standard frontline therapy. At the time, this was devastating news for the neuroblastoma community, with immunotherapy being the primary novel approach that was offering hope for children.
Solving Kids’ Cancer UK immediately launched an appeal against this decision, knowing that it would have such a detrimental impact on children and their families. The appeal was based on two grounds: That the decision was against the European Convention on Human Rights as it pertained to children, and that it was unreasonable based on the evidence presented.
At the appeal meeting in September 2016, representatives from SKC UK presented a number of arguments and re-evaluated evidence justifying the use of dinutuximab. These were largely based on the unique aspects of neuroblastoma and the young patient population it affects, and that these were not properly considered in NICE’s standard appraisal process. In November 2016, the appeal was ruled successful on both grounds.
In February 2017, before any further NICE hearings that would have led to a final recommendation, United Therapeutics withdrew Unituxin© from the European market due to supply issues. Because of this, the NICE appraisal was suspended indefinitely and was permanently discontinued in 2022.
NICE appraisal of dinutuximab beta
Following the withdrawal of dinutuximab, a NICE appraisal for a newer anti-GD2 antibody called dinutuximab beta was launched in March 2017. Dinutuximab beta is marketed by Recordati Rare Diseases under the name Qarziba©, and at the time was being investigated in several trials across Europe, showing comparable effectiveness to Unituxin©.
Solving Kids’ Cancer UK was engaged with NICE from the beginning of the appraisal process following their influence on the decision process for dinutuximab. In May 2018, NICE published its draft guidance which recommended that dinutuximab beta should not be used in standard treatment. As before, the neuroblastoma community was extremely disappointed and concerned at this outcome and the impact it could have on children.
Solving Kids’ Cancer UK became heavily involved in the consultation period that followed, providing extensive supporting evidence and testimonies including a commentary by Chair of Trustees Nick Bird. The information drew largely on that which was presented during the previous appeal regarding dinutuximab, again highlighting the unique challenges of neuroblastoma that were crucial to consider.
In July 2018 NICE published the final appraisal decision (FAD) for dinutuximab beta recommending that it should be introduced to standard treatment, much to the relief of the charity and the entire neuroblastoma community. Since then, children have been able to access dinutuximab beta through the NHS, and this is now recognised as an essential treatment for high-risk neuroblastoma. The decision was heavily informed by the evidence presented by Solving Kids’ Cancer UK and other experts, and NICE cited that the decision was “taking into account the uncaptured health-related benefits, the rarity and severity of the disease and the potential lifetime benefit for children with neuroblastoma, dinutuximab beta can be recommended for high-risk neuroblastoma”.
Impact of our involvement
Without the commitment and expertise of the Solving Kids’ Cancer UK team throughout both NICE consultations on anti-GD2 antibody therapy, children in the UK would likely not have access to immunotherapy treatment as standard of care. Given the evidence that it can considerably improve the outcomes for children with high-risk neuroblastoma, this would have been a tremendous setback in successfully treating as many children as possible.
The uncertainty around accessing immunotherapy, which represented a lifeline for many families at the time, was very distressing throughout the extensive consultation, giving just a glimpse into what could have been a very different reality for families today.
On a broader scale, the continuous dialogue between Solving Kids’ Cancer UK and NICE has set a new precedent for evaluating new medicines for children’s cancers. In both instances, NICE’s U-turn was significantly influenced by the argument that introducing novel therapies for children with cancer comes with nuances that are not accounted for in many of their procedures, that were founded in more clear-cut clinical cases.
As research into neuroblastoma and other children’s cancers develops, and more novel treatments are discovered, there are many learnings that can be taken from this story that can influence a better approach to bringing better treatments to children as rapidly as possible.
Our latest news

Joint statement in response to NICE’s decision to approve anti-GD2 antibody therapy dinutuximab beta
Families now know their very sick child will be able to have the best treatment that gives the greatest chance of long-term survival here in the UK.

Anti-GD2 antibody therapy update, May 2018
Following the recent provisional decision by the National Institute for Health and Care Excellence (NICE) not to approve dinutuximab beta (Qarziba™) for use on the NHS in England and Wales, we have updated our briefing document.

Joint statement in response to NICE’s preliminary decision on Anti-GD2 antibody therapy dinutuximab beta
From Solving Kids’ Cancer, Neuroblastoma UK, the Children’s Cancer and Leukaemia group, JACK, The Bradley Lowery Foundation, Hugs from Henry, Christopher’s Smile, Niamh’s Next Steps and Smile with Siddy.