Optimizing Immunotherapy for chemo-resistant neuroblastoma
| Study name | Optimizing Immunotherapy for chemo-resistant neuroblastoma |
| Study type | Preclinical study |
| Principal investigator(s) | Dr Robbie Majzner |
| Institutions | University College London (UCL), The Institute of Cancer Research (ICR), Stanford University |
| Partners | Joining Against Cancer In Kids (J-A-C-K), Merryn Lacy Trust, Oscar Knox Fund, Rupert's Revenge, Zoé4Life |
| Total awarded | £500,000 |
| Solving Kids’ Cancer UK contribution | £150,000 |
Overview
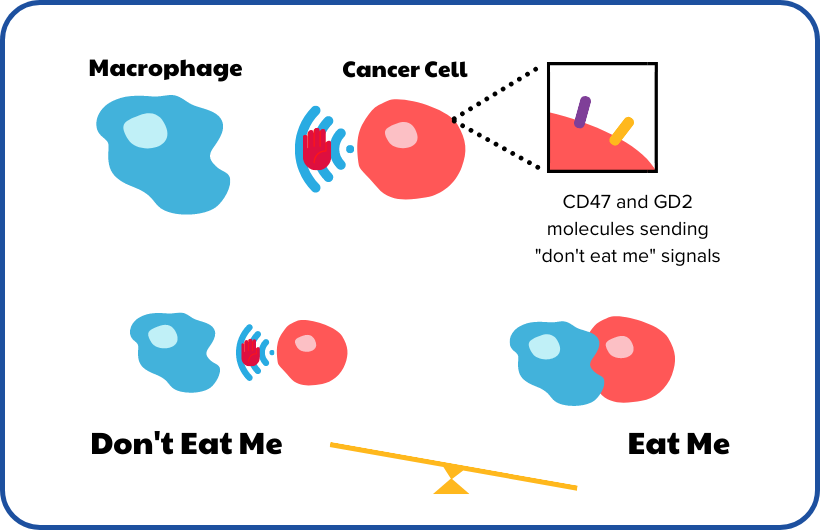
Researchers at Stanford University, ICR and UCL, led by Dr Robbie Majzner will investigate how to improve chemoimmunotherapy for patients with primary refractory neuroblastoma, that is resistant to most therapies. Chemoimmunotherapy combines chemotherapy and immunotherapy to unleash a patient's immune response to neuroblastoma cells. Anti-GD2 antibodies have been one of the key clinical advances in recent years, which have led to increased survival rates. In combination with existing chemotherapy agents, it has led to a good clinical response in children whose disease does not respond to chemotherapy alone. The advantage of this treatment is that it does not rely on killing tumour cells with specific drugs, but instead helps the immune system to kill the cells.
This works by exploiting the mechanism by which neuroblastoma survives in the body at a molecular level. Neuroblastoma cells have molecules on their surface, called GD2 and CD47, which trick the immune system into not destroying them by sending so-called ‘don’t eat me’ signals. The team plans to run preclinical studies to optimise current anti-GD2 chemoimmunotherapy and introduce a new anti-CD47 agent to this regimen, in hopes to block the ‘don’t eat me’ signal and switch on ‘eat me’ signals. This will allow immune cells to function as they should and destroy neuroblastoma cells. The end goal is to build a successful combination therapy with chemotherapy, anti-GD2 agents and anti-CD47 agents, which can be taken to a clinical trial.
Impact
This study helps address the unmet needs of children diagnosed with primary refractory neuroblastoma, who currently have very few options for innovative treatments. The development of a clinical trial specific to this sub-type of disease, and with a treatment that eradicates toxic chemotherapies which are not effective, will be ground-breaking for this group of patients who are high priority for advancements in the clinic.
"Reaching those in the neuroblastoma community whose needs are profoundly unmet is of the upmost importance for Solving Kids’ Cancer UK and the direction of our research portfolio. This work is integral in doing that for children with chemo-resistant disease where options are extremely limited. We are very excited to see the important progress that can be made in this area by Dr Majzner and his team."
Gail Jackson, CEO Solving Kids' Cancer UK